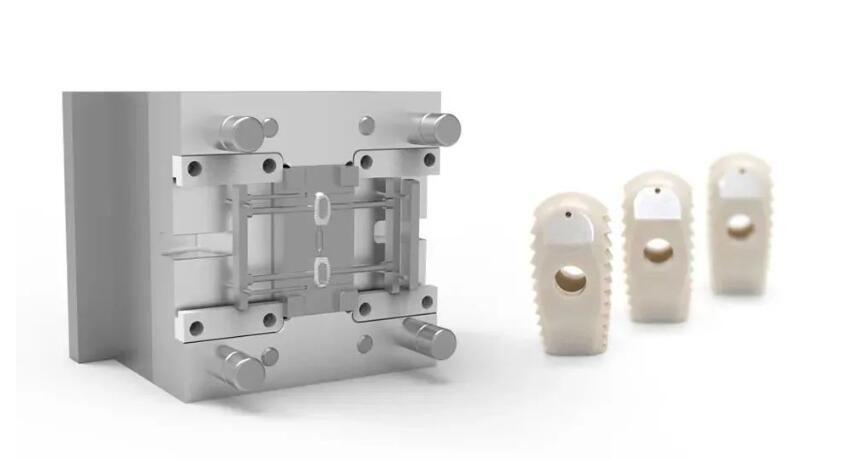
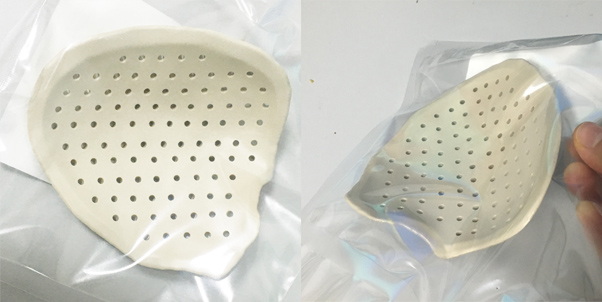
Puerto de infusión de PEEK

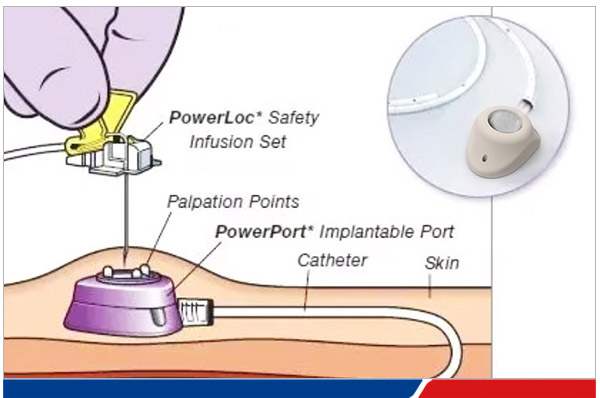
What is a PORT
The infusion port (PORT), also known as the implantable venous catheter system, is a closed venous infusion system that can be completely implanted in the body. 80% of them are used for cancer treatment and infusion of chemotherapy drugs. They can also be used for intravenous infusion, parenteral nutrition, blood products, blood specimens, etc. PORT can be used for the infusion of all drugs of different natures.
A fully implantable infusion port, referred to as an infusion port, is a closed venous infusion system that is completely implanted subcutaneously and remains in the body for a long time. It includes a catheter part located in the superior vena cava and an injection seat implanted subcutaneously. It is a method of inserting a catheter through a puncture of the internal jugular vein or subclavian vein, allowing the catheter tip to enter the superior vena cava, inserting an infusion port seat in the subclavian fossa, and connecting the infusion port seat to the catheter. It is a long-term venous channel for intravenous infusion in patients. Depending on the implantation site, it can be divided into two types: chest wall infusion port and arm infusion port, which can be used for drug infusion, fluid replacement, nutritional support, blood transfusion, blood sample collection, etc.
Implant-grade PEEK materials have excellent physical and chemical properties, radiolucency and biocompatibility. They have successfully partially replaced metals and other high-performance resins in the fields of orthopedic tumors, plastic surgery, spine, knee joints and craniofacial repair.
AKSOPEEK Advantages
What are the advantages of AKSOPEEK infusion port over traditional infusion port
1. Good biocompatibility;
2. Low density, lighter and more comfortable to wear than metal PEEK infusion ports;
3. PEEK has good thermal insulation and is not easy to accumulate heat when bathing or sunbathing;
4. PEEK itself has antibacterial properties, so it is not easy to cause infection in the case of long-term implantation, thus reducing complications;
5. PEEK has good corrosion resistance and is not easy to react with the input liquid medicine;
6. PEEK has good radiation resistance and will not be affected by radiotherapy or chemotherapy rays.
Company advantages
1. As one of the earliest domestic companies engaged in the research, development, and production of PEEK materials, Jiangsu Junhua pays great attention to the application of PEEK in the medical industry and has been committed to the research, development, and production of medical implant-grade PEEK materials. It can supply implant-grade PEEK material particles, rods, plates, and 3D printing PEEK filaments.
2. After 16 years of production and technology accumulation, the company started from the source of polymerization and established Shandong Junhao High Performance Polymer Co., Ltd. to develop polymer implantable PEEK raw materials.

3. A GMP refining production workshop was established in accordance with the production standards for medical implant materials, and refining and purification equipment for implant-grade AKSOPEEK polymerization was developed, which effectively solved the problem of excessive heavy metal ion content in implant PEEK materials.
4. The company started pilot production of implantable-grade PEEK materials in 2020. In the same year, it conducted third-party tests on various biological aspects of the materials in accordance with ISO10993 testing standards, and all test results met the requirements.
5. In 2021, Junhua sent the first batch of mass-produced implantable-grade AKSOPEEK to a third party for inspection in accordance with the national requirements for implantable PEEK materials «YY/T 0660-2008 Standard Specification for Polyetheretherketone (PEEK) Polymers for Surgical Implants», and obtained a complete third-party test report on implantable PEEK materials to ensure that the material can be effectively replaced in terms of physical and chemical properties and biology.
Implant-grade PEEK: As the name implies, PEEK materials that can be used to prepare «implant devices» are called implant-grade PEEK. This type of material is commonly used in orthopedic implant consumables (such as intervertebral fusion devices, ligament repair anchors, joint interface screws), neurosurgery patches (such as artificial skulls, maxillofacial bones), and cardiovascular products (such as heart valves, pacemaker housings, etc.). In recent years, modified PEEK materials have begun to be widely used in oral implants, traumatic bone plates and other products with high mechanical performance requirements. In addition to meeting the biocompatibility of medical-grade PEEK, implant-grade PEEK should also have more stringent biosafety requirements, such as systemic toxicity, genotoxicity, carcinogenicity, blood compatibility, and implantation reactions, and must also comply with the requirements of «YY/T 0660-2008 Standard Specification for Polyetheretherketone (PEEK) Polymers for Surgical Implants».
AKSOPEEK
China’s implant-grade PEEK materials mainly rely on imports. Based on market feedback and national R&D project needs, Junhua PEEK has launched the domestic implant-grade PEEK material brand AKSOPEEK series of products.
Based on the present and looking to the future. In 2018, Changzhou Junhua Medical officially launched the implantable PEEK project. At present, it has obtained complete third-party reports on biology, physicochemistry, aging, etc. In order to better standardize implantable PEEK, it has added implantable AKSOPEEK series grades.
1) AKSOPEEK Natural : Implant-grade PEEK pure material
2) AKSOPEEK HA : Hydroxyapatite improves the biocompatibility of implantable PEEK materials
3) AKSOPEEK CF : Chopped carbon fiber implant grade PEEK material
4) AKSOPEEK LCF : Continuous carbon fiber implant grade PEEK material
Application Scenario
The domestically produced medical implant-grade PEEK material AKSOPEEK has excellent physical and chemical properties, and its radiotransparency, biocompatibility and human bone matching are no less than similar foreign products, achieving import substitution of PEEK materials in the fields of orthopedic tumors, plastic surgery, spine, knee joints and craniofacial repair.