Más de 18 años de experiencia en producción e I+D de aplicaciones PEEK
Aprobó la certificación empresarial nacional de alta tecnología en 2015 y fue calificada como una nueva empresa "pequeño gigante" especializada a nivel nacional en 2022.
18
Más de 18 años de experiencia en la industria
500
Equipo profesional de más de 500 personas.
3
Las ventas anuales superan los 300 millones
30000
Más de 30.000 soluciones
JunHua PEEK
JunHua PEEK se centra en la investigación, el desarrollo y la producción de aplicaciones de perfiles y resinas plásticas de ingeniería especiales de alto rendimiento como PEEK (polieteretercetona), y ha formado una línea con polimerización de resina de materia prima PEEK y granulación modificada. y productos terminados Una cadena industrial completa de moldeo por inyección y mecanizado de piezas, y extrusión continua de perfiles como placas, varillas, tubos y láminas.
¿No encuentra el producto PEEK ideal para su industria?
Guía de selección de materiales, diseño de nuevos materiales compuestos, medición 3D del producto...
¿Por qué elegirnos?
Por qué más de 1000 socios en todo el mundo eligen Junhua
Utilice las principales ventajas técnicas para aportarle más valor
Nuestras ventajas
Ventajas técnicas
-
18+ years of experience in PEEK application R&D and production;
-
40 dedicated production lines for continuous extrusion of high-performance special engineering plastic profiles;
-
It passed the national high-tech enterprise certification in 2015 and was approved as a national specialized and innovative "little giant" enterprise in 2022.
-
The "PEEK Profiles and Products Enterprise Standard" was formulated.
-
The Wujin District Engineering Technology Research Center was established in 2014, and the Jiangsu Province (Junhua) High-Performance Special Engineering Plastics Engineering Technology Research Center was completed in 2022;
-
Continuous technological innovation has accumulated proprietary technologies: 12 innovations and 27 utility models;
-
The company has maintained long-term technical exchanges and cooperation with Jilin University and Nanjing University of Chemical Technology;
-
The efficient R&D technical team is constantly conducting application research and development of PEEK.
Ventaja de calidad
-
Established a strict IATF16949 quality assurance system and continued to improve;
-
Strict inspection: unqualified raw materials will not be put into production, unqualified semi-finished products will not be transferred, and unqualified finished products will not leave the factory;
-
Professional and efficient R&D technical team: provide technical support to customers and fully protect their interests;
-
Authoritative testing and certification: FDA food grade certification, SGS quality system certification, ROHS testing and other third-party certifications;
-
Advanced testing equipment: ultrasonic testing instrument, electronic mechanical testing machine with high and low temperature functions, friction and wear testing machine, high temperature melt index tester, three-coordinate measuring instrument, X-ray foreign body detector, etc.
-
Complete after-sales service system: product quality, product performance and customer follow-up services.
Ventajas de producción
-
Eighteen years of experience in PEEK application research and development and production;
-
At the same time, it has the R&D and production capabilities of the complete industrial chain of PEEK raw material resin polymerization and modified granulation, continuous extrusion molding of plate and rod profiles, and injection molding machine processing of finished parts;
-
There are many types of products with various specifications, and they are always in stock;
-
Unique production and testing equipment: 40 profile extrusion production lines, four-axis and five-axis CNC machining, turning and milling compound, three-coordinate measuring instrument, X-ray foreign body detector, mechanical testing machine, infrared spectrometer, thermogravimetric analyzer, and differential scanning calorimeter;
-
The company currently has four major subsidiaries, namely Shandong Junhao, Changzhou Junhua Medical, Changzhou Junhang and JunDe HPP GmbH, with eight business divisions, namely Profile Extrusion Division, Analytical Instrument Division, Aviation Division, Gear and Seal Transmission Division, Electronic Semiconductor Division, Oil Production and Petrochemical Division, and 13 affiliated workshops, which support and cooperate with each other for common development.
-
Complete hardware facilities: 16,000 square meters of standardized office and production workshops, 300 square meters of enterprise product showrooms.
Un socio confiable
La elección de más de 1000 empresas de todo el mundo, su socio de confianza









Servicio y Soporte
Junhua es una empresa líder especializada en materiales de polieteretercetona. Tiene una cadena industrial completa de polieteretercetona y está comprometida a brindar soluciones integrales de plásticos de ingeniería de alto rendimiento a clientes globales. Junhua puede ayudar a los clientes a desarrollar nuevas aplicaciones industriales de productos de polieteretercetona basadas en las necesidades de los clientes y a mejorar el rendimiento de los productos plásticos de ingeniería existentes.

Junhua PEEK
Diseño y procesamiento
Junhua ofrece diferentes soluciones de moldeo de productos, como moldeo por inyección (incluido el diseño de moldes y fabricación interna), mecanizado y extrusión de acuerdo con el diseño de su producto para optimizar sus costos. Obtener Servicios
Junhua PEEK
Selección de materiales
Junhua se especializa en materiales PEEK. Tenemos docenas de grados de PEEK comunes y personalizados para que los clientes elijan. Háganos saber los requisitos de diseño de su producto, como requisitos de rendimiento mecánico, temperatura de funcionamiento, constante dieléctrica, etc. Le recomendaremos PEEK o PEEK adecuado. otros plásticos de ingeniería de alto rendimiento para usted. Obtener Servicios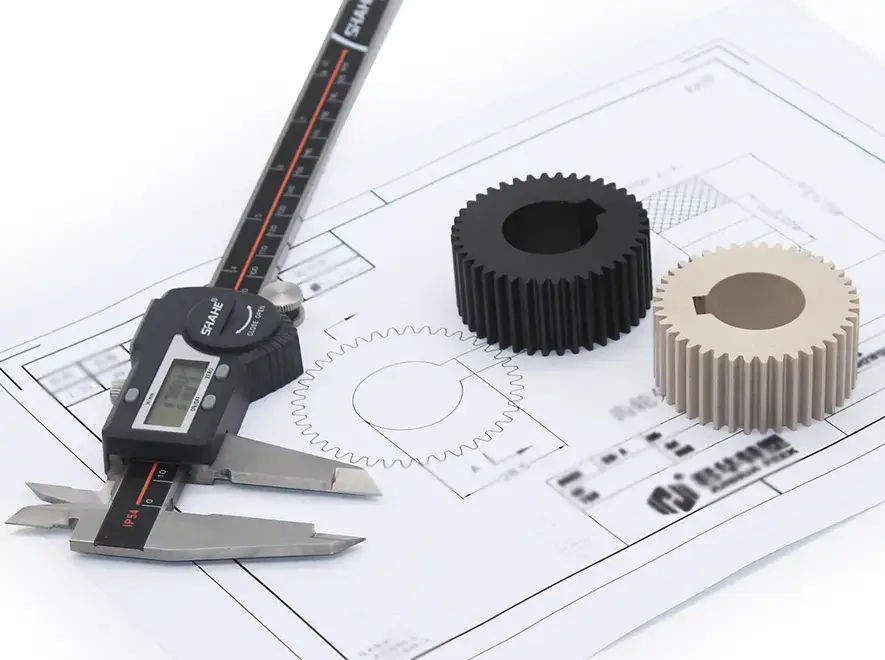
Junhua PEEK
Topografía
Junhua Co., Ltd. tiene muestras para topografía y cartografía. Cuenta con equipos avanzados, como instrumentos de medición de imágenes (con una precisión de hasta 0,001 mm), instrumentos de medición de tres coordenadas (con una precisión de hasta 0,003 mm) y. Instrumentos de medición de planitud para garantizar que se proporcionen dibujos precisos a los clientes. Obtener Servicios
Junhua PEEK
Muestreo
Junhua enviará muestras a los clientes para pruebas de ensamblaje de acuerdo con la cantidad requerida por los clientes. Si la primera muestra pasa, nuestra empresa llevará a cabo la producción en masa y las pruebas de acuerdo con las muestras confirmadas. Si la primera muestra falla, nuestra empresa ajustará el producto. proceso de diseño o producción de acuerdo con los comentarios del cliente hasta que la muestra pase la prueba del cliente y luego envíe la muestra nuevamente. Obtener Servicios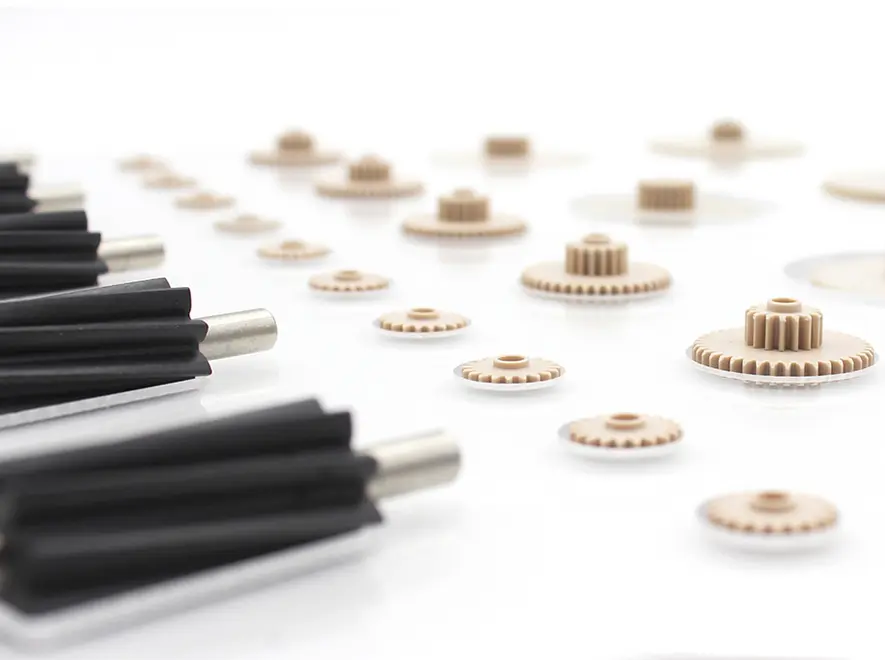
Junhua PEEK
Producción en masa
Una vez aprobada la muestra, Junhua solidificará el proceso de producción del producto y emitirá una guía de operación de producción para referencia del departamento de producción desde la adquisición de materias primas, la confirmación del primer producto, el control de temperatura durante el proceso de producción y la calidad. Inspección, Junhua controla cada pequeño detalle para garantizar la calidad de los productos por lotes. Obtener Servicios
Junhua PEEK
Control de calidad
Junhua implementa estrictamente el sistema de gestión de calidad ISO9001:2008 y controla estrictamente la calidad de cada lote de productos. Durante el proceso de producción, los operadores realizan autoinspecciones y el personal de control de calidad realiza inspecciones aleatorias y las registra una vez finalizados los productos. inspección completa o inspección aleatoria de todo el lote de productos terminados y proporcionar a los clientes informes COA, informes de inspección de tamaño y otros informes de inspección requeridos por los clientes. Obtener Servicios
Junhua PEEK
Mejorar
Junhua implementa un sistema de propuestas de mejora con plena participación, promueve el ciclo PDCA, mide y registra cada lote de productos y optimiza continuamente el diseño de productos o las soluciones de procesamiento basándose en la experiencia de casos similares de clientes. Proporciona a los clientes mejores soluciones de productos PEEK a través del objetivo. tasa de logro, análisis de factores de influencia y verificación del plan de mejora. Obtener Servicios
Junhua PEEK













